COV-X is a novel first-in-class small molecule oral pan-coronavirus antiviral candidate
- COV-X outperforms Pfizer’s approved antiviral in a pre-clinical mouse SARS-CoV-2 efficacy trial
- Unlike many competitors, COV-X blocks a unique key protein and has potential for broad spectrum efficacy against new coronaviruses with pandemic potential
- Emerging resistance to MPRO inhibitors, such as Pfizer’s antiviral, is driving the urgent need for new therapeutic options to treat patients with serious coronavirus infections
Alderley Park, Cheshire, U.K. Infex Therapeutics, a leading anti-infectives specialist, announces the nomination of a clinical candidate for its in-house developed COV-X programme. The candidate for COV-X, the Company’s novel first-in-class small molecule oral pan-coronavirus PLPRO inhibitor, was selected following excellent in vivo efficacy data in a murine SARS-CoV-2 model that showed a near complete reduction in SARS-CoV-2 viral load in the lungs and an outstanding safety profile when compared to Pfizer’s approved MPRO inhibitor nirmatrelvir.
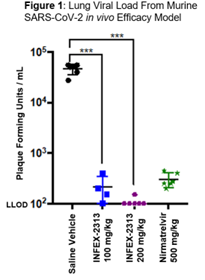
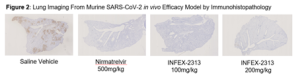
The pre-clinical study aimed to assess the efficacy and safety profile of COV-X against MPRO inhibitor nirmatrelvir and a control group (saline vehicle only). Two dosing regimens were utilised; 100 mg/kg and 200 mg/kg. Viral load was then measured by plaque assay and lung damage was monitored using lung imaging. In five of the six samples dosed with COV-X at 200 mg/kg, viral load in the lungs was below the limit of detection (Figure 1). Even at doses five times lower than nirmatrelvir, COV-X provided protection from lung damage (Figure 2), with treated groups showing a significant reduction in inflammatory cytokines; IL-6 by 80% and CCL2 by 94%. COV-X also demonstrated a superior DMPK profile to nirmatrelvir.
Unlike nirmatrelvir, COV-X targets the key coronavirus PLPRO enzyme. PLPRO is essential for viral replication and evasion of host immune response, and is conserved across SARS-CoV-2, SARS-CoV-1, MERS and all related coronavirus variants. As a result, COV-X has the potential for broad spectrum efficacy against new coronaviruses or variants thereof with pandemic potential.
Furthermore, development of new variants of SARS-CoV-2 that are resistant to nirmatrelvir have been reported widely in recent weeks, with further cross resistance to other drugs in the MPRO class. Infex’s COV-X programme offers an alternative for treatment of resistant strains, as a stand-alone therapy, or in combination with MPRO drugs.
With a pre-clinical candidate nominated with back-up compounds, COV-X is currently undergoing CTA/IND-enabling studies to provide a comprehensive safety profile ahead of clinical stage development. Given the strong pre-clinical data, Infex is accelerating its development towards partnerships and commercialisation.
Dr Peter Jackson, CEO of Infex Therapeutics, commented: “With global SARS-CoV-2 vaccines showing increasing weakness against new variants, it will be essential to develop effective broad-spectrum antivirals that can future-proof against new coronaviruses with pandemic potential. Following encouraging pre-clinical data in comparison with Pfizer’s MPRO inhibitor nirmatrelvir, we have high hopes for our nominated candidate for our COV-X programme. COV-X, our first-in-class small molecule oral pan-coronavirus PLPRO inhibitor, demonstrated a superior efficacy and DMPK profile to nirmatrelvir, Pfizer’s approved blockbuster COVID prophylactic, and its ability to reduce viral load in the lungs is key to minimising the chances of long term viral conditions, such as long COVID. Given COV-X’s outstanding profile and potential value, we are now seeking a global pharmaceutical partner to accelerate its development towards commercialisation.”
